Does Carboxylic Acid Have Higher Boiling Point Than Alcohol
The hydrogen bonds are not broken completely even in the vapour phase. Asked May 7 2018 in Chemistry by paayal 147k points neet.
16 2 Properties Of Carboxylic Acids Ppt Download
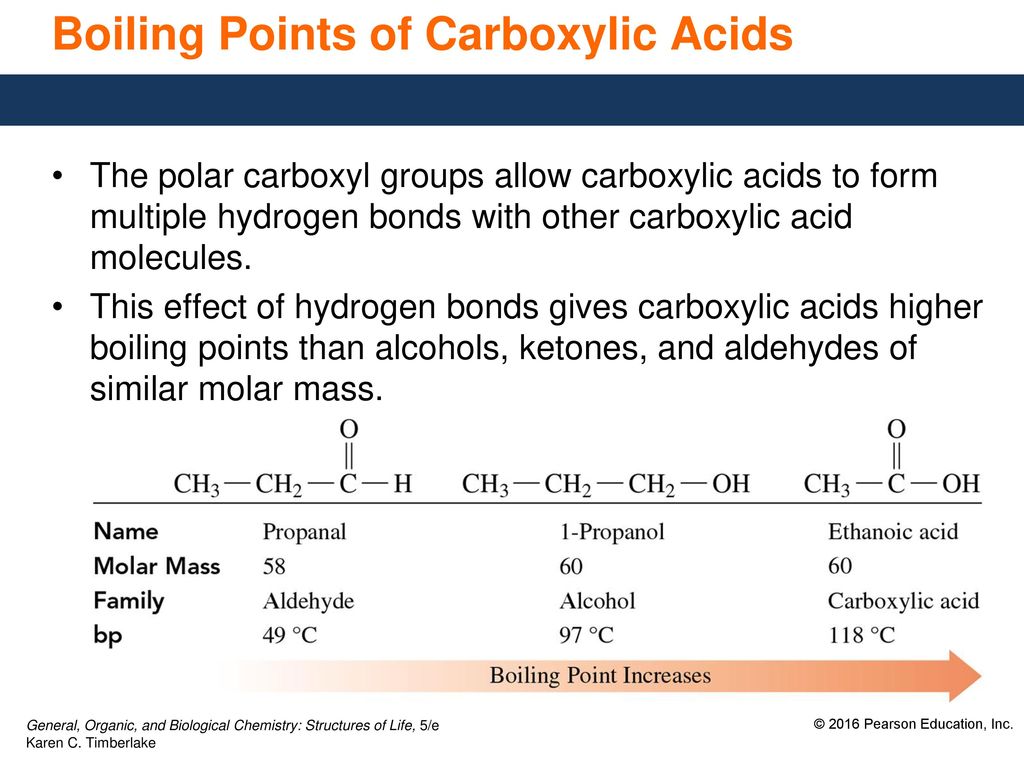
Carboxylic acids have higher boiling points since the formation of dimers give them an extra stability.

. Carboxylic acids have higher boiling points then alcohols due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding. The acidity of carboxylic acids arise as the H in carboxylic acid can be released as a proton. The hydrogen bonds are not broken completely even in the vapour phase.
This proton-releasing causes the pH of the system to be increased indicating an acidic behavior. Carboxylic acids similar to alcohols can form hydrogen bonds with each other as well as van der Waals dispersion forces and dipole-dipole interactions. They have the same molecular mass 60.
Correct option is C Carboxylic acids have higher boiling points than aldehydes ketones and given alcohol of comparable molecular mass. The hydrogen bonds are not broken completely even in the vapour phase. Yes the carboxcyllic acid molecules form to hydrogen bonds with each other whereas alcohol molecules only.
Lets take an example of ethanoic acid and propanol. Because of their ability to form intermolecular hydrogen bonding carboxylic acids have high boiling points as compared to the corresponding alcohol. Carboxylic acid have higher boiling point than aldehydes ketones and even alcohols of comparable molecular mass.
This is because of formation of intermolecular H. On comparing it with the corresponding alcohol carboxylic acid has a higher boiling than the corresponding aldehyde ketone and even alcohols of the comparable molecular masses. The boiling point of carboxylic acids are higher than the boiling points of alcohols.
Carboxylic acids have even higher boiling points then alkanes and alcohols. The boiling points of carboxylic acids are higher than the corresponding alcohols because. Carboxylic acids have higher boiling points than aldehydes ketones and even alcohols of comparable molecular mass.
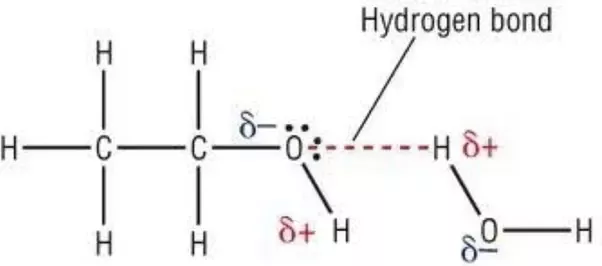
Hydrogen bonds formed in carboxylic acids are stronger than those in alcohols. Carboxylic acids have higher boiling point than alcohols due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding. Even the simplest carboxylic acid formic acid boils at 101 C 214 F which is considerably higher than the boiling point of ethanol ethyl alcohol C 2 H 5 OH which boils at 785 C 173 F although the two have nearly identical molecular weights.
The hydrogen bond formed by the carboxylic acids are stronger than those in alcohols because OH bond in COOH is more strongly polarised due to the presence of electron withdrawing carboxy group in adjacent position then. Hence carboxylic acids have higher boiling points than alcohols. Carboxylic acids have higher boiling points then alcohols due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding.
The boiling point of the ethanoic acid is text391K whereas that of. Due to this Carboxylic acids exist as a dimer Relatively large amount of energy is required to be supplied to break this strong bonding Hence boiling point of. The boiling points of carboxylic acids increases as the molecules get bigger.
The OH bond in COOH is more strongly polarised than the OH bond of alcohols. Asked Dec 26 2018 in Aldehydes Ketones and Carboxylic Acids by monuk 680k points. Carboxylic acids have higher boiling pint than aldehyde keton and even alcohols of comparable molecular masses.
Even the simplest carboxylic acid formic acid boils at 101 C 214 F which is considerably higher than the boiling point of ethanol ethyl alcohol C2H5OH which boils at 785 C 173 F although the two have nearly identical molecular weights. Carboxylic acids have a high boiling point because of their ability to form intermolecular hydrogen bonds.

Carboxylic Acid Have Higher Boiling Points Than Aldehydes Ketones And Even Alcohol Of Youtube

Why Do Carboxylic Acids Have Higher Boiling Points Than Alcohol Aof Comparable Molecular Mass
Why Does Carboxylic Acid Have A Higher Boiling Point Than Alcohols With The Same Molecular Weight Quora
No comments for "Does Carboxylic Acid Have Higher Boiling Point Than Alcohol"
Post a Comment